The deletion 'cassette' used to replace each yeast gene was constructed in a sequential two-step PCR reaction using two pairs of primers (Figure 1). In the first amplification, 74bp UPTAG and 74bp DNTAG primers consisting of (5' to 3'): 1) 18 bp of genomic sequence that flank either the 5' or 3' end of the ORF (directly proximal and distal to the start and stop codons respectively), 2) 18 and 17 bp of sequence common to all gene disruptions (for amplifying the 'molecular bar-codes' in a PCR;U1: 5'-GATGTCCACGAGGTCTCT-3' or D1: 5'-CGGTGTCGGTCTCGTAG-3'), 3) a 20 bp unique sequence (the 'molecular bar-code' TAG) and 4) 18 and 19 bp of sequence, respectively, homologous to the KanMX4 cassette (U2: 5'- CGTACGCTGCAGGTCGAC-3' or D2: 5'-ATCGATGAATTCGAGCTCG -3') the other priming site for amplifying the 'molecular bar-codes'. The KanMX gene expressed from a constitutive promoter allowed for selection of transformants. The resulting product of the first amplification is a molecularly bar-coded gene-specific deletion cassette. In the second PCR reaction, two ORF specific 45-mer oligonucleotides (UP_45 and DOWN_45) were used to extend the ORF specific homology to 45 bp, thereby increasing the targeting specificity (during mitotic recombination) of the gene disruption cassette. The presence of two tags ('UPTAG' and 'DNTAG') increases the quality of the hybridization data from the oligonucleotide arrays by adding redundancy (approximately 3.2 % of the strains harbor only one unique UPTAG sequence).
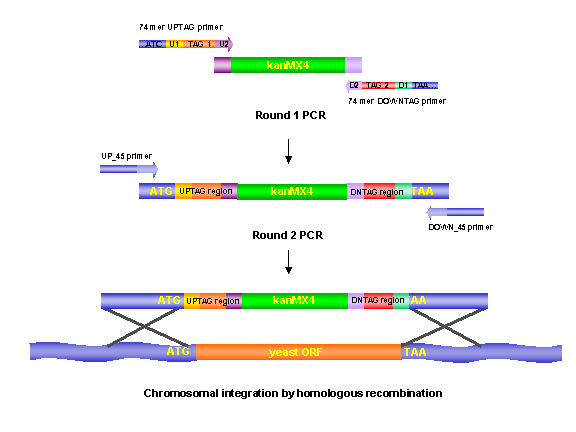
Figure 1
Software routines that automate primer selection were written at the Stanford Genome Technology Center (SGTC) and were based on annotated sequence information from SGD . ORF and sequence data were updated from the site over a 3 year period. We did not attempt to delete 6.1% of the genes because unique primers could not be chosen in the 45bp regions flanking these ORFs. The criteria for this cutoff was based on a BLASTN search where E = 10 and the mismatches allowed = 0. Software routines that generated the tags used in the above construction of the deletion cassette were developed in collaboration with Affymetrix (Santa Clara, CA).
All primers were synthesized at the SGTC on an Automated Multiplex Oligonucleotide Synthesizer (AMOS) in 5-10 nM amounts in batches of 96 using standard phosphoamidite chemistry. Deletion cassettes were amplified at Stanford and distributed for subsequent transformation of yeast (along with the confirmation primers) to the several labs participating in this project. Greater than 99% of primers successfully generated the deletion cassette modules. The remaining < 1% were successful when resynthesized.
For the majority of the deletions (70%) the gene disruption cassette was introduced into diploid yeast cells (MATa/a his3D1/his3D1 leu2D0 /leu2D0 lys2D0/LYS2 MET15/met15D0 ura3D0 /ura3D0 ) using a standard lithium acetate transformation protocol followed by selection of colonies on G418-containing agar plates. The resulting transformants were sporulated (see below) and haploid MATa and MATa deletion mutants were recovered from the tetrads. The remaining 30% of the gene disruptions were constructed by direct transformation of MATa and MATa haploids. To generate homozygous diploids, two independently constructed haploid mutants were mated.
A second attempt was made to delete ORFs that were not successfully deleted in the first attempt. The success rates for Round 1 and 2 deletion attempts were 92% and 74% respectively. Genes not successfully deleted after the second round were attempted again using an additional pair of longer (63 mer) primers (UP_90 and DOWN _90) that extended the sequence flanking the ORF to be deleted to 90 bp. About 10% of the collection was attempted using such primers; the success rate for these disruptions (rounds 3 and 4) was >97%.
During construction of the YKO collection, two problems had to be avoided.
First, a significant number of the primary heterozygous diploid transformants
carried recessive mutations unlinked to the gene deletion. These were apparent
in tetrads with lethal or slow growth mutations that segregated independently
of the KanMX gene. These mutations are likely induced during the
DNA transformation procedure, which is known to be mutagenic. Mutants that
behaved this way (about 6.5% of the heterozygous KanMX-containing
primary transformants, estimated from a sample of 819 mutants), were discarded.
Second, a substantial fraction of the haploid deletion mutants were found
to carry a wild-type copy of the gene to be deleted, in addition to the
correct deletion mutation (confirmed by the appropriate PCR tests). Such
cases are likely due to aneuploidy: a duplication of all or part of the
chromosome (B. Dujon, pers. comm., and our observations described below).
They comprised about 1% of the heterozygous KanMX-containing primary
transformants (estimated from a sample of about 1300 mutants). They were
identified through a PCR reaction (described below) and discarded.
The correct replacement of the gene with KanMX was verified in the
haploid mutants (for nonessential genes) or in the heterozygous diploid
mutants (for essential genes) by the appearance of PCR products of the expected
size using primers that span the left and right junctions of the deletion
module within the genome (Figure 2). Four ORF-specific
primers were chosen using a modified version of the 'PRIMER'
program (Whitehead Institute). The "A" and "D" primers were positioned 200-400
bp from the start and stop codons of the gene, respectively. The "B" and
"C" primers were located within the coding region of the ORF and, when used
with the A or D primers, gave product sizes between 250-1000 bp. The "KanB"
(5'-CTGCAGCGAGGAGCCGTAAT-3') and "KanC" (5'-TGATTTTGATGACGAGCGTAAT-3') primers
are internal to the KanMX4 module. Primers lengths ranged from 17 to 35
nucleotides; their Tm was 65 +2° C; they had 30-70% G/C content. The junctions
of the disruption were verified by amplification of genomic DNA using primers
"A" and "KanB" (reaction 1) and primers "KanC" and "D" (reaction 2) (Figure
2b). Deletion of the ORF was verified by the absence of a PCR product
using primers "A" with "B" (reaction 3) and "C" with "D" (reaction 4) (Figure
2a). Finally, each deletion mutant was checked for a PCR product of
the proper size using the "A" and "D" primers flanking the gene (reaction
5). For a deletion to become part of the collection it had to pass the test
of reactions 3 and 4 and two of the three tests of reactions 1, 2 and 5.
In the case of heterozygous strains a successful deletion was indicated
by the additional appearance of a wildtype-sized PCR product in reactions
3, 4 and 5. Lastly, each deletion mutant was checked for the appropriate
auxotrphic markers and mating capabilities.
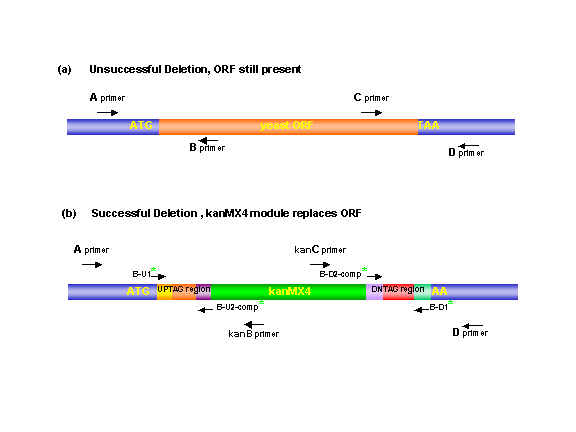
Figure 2
Diploid yeasts were sporulated by patching a freshly grown culture from YPD plates onto freshly prepared (less than one week old) GNA presporulation plates (5% dextrose, 3% Difco nutrient broth, 1% Difco yeast extract, 2% Difco bacto-agar; and grown no more than 24 hours at 30°C. (Subculturing again on GNA plates increases sporulation, but is not necessary.) One colony equivalent of cells was transferred to 2ml of liquid sporulation medium and incubated on a roller wheel for 1 day at 25°C followed by 3-5 days at 30°C. This protocol yields up to 30% sporulated diploids
Screening the mutants for altered cell morphologies:
Homozygous deletion strains were grown individually at 30°C with 0.8ml liquid YPAD in 96-well microtiter plates with shaking; each well contained one 3.5 mm glass bead to facilitate mixing. The cells were grown to stationary phase, then diluted and grown to mid-log-phase (at least six generations). Cells were fixed by the addition of formaldehyde to a final concentration of 3.7%, incubated for one hour at 30°C, washed with PBS, resuspended in PBS, and examined by phase-contrast and differential interference contrast microscopy.
The mutants were scored using a scale of 1-4 (see table and legend below)
and grouped into seven classes: elongated, round, small, large, football-shaped
or pointed, clumpy and other. "Other," being defined as a deletion
mutant having 3 or more distinct phenotypes. Cells that exhibited a "chain"
or "branched" phenotype were also categorized as "other."
To see a list of all the strains screened, see the Cell
Morphology Screen Table. (Strains that are not yet screened are designated
as "ND.")
Morphological Screen Results
|
Phenotype
|
Score*
|
No.
|
%
|
%
|
|
Elongate
|
2 <3 |
62 57 |
1.41 1.30 |
2.70
|
|
Round
|
2 <3 |
104 98 |
2.36 2.23 |
4.59
|
|
Small
|
2 <3 |
87 75 |
1.98 1.70 |
3.68
|
|
Large
|
2 <3 |
69 76 |
1.57 1.73 |
3.29
|
|
Football/Pointed
|
2 <3 |
53 59 |
1.20 1.34 |
2.54
|
|
Clumpy |
2 <3 |
10 14 |
0.23 0.32 |
0.55
|
|
Other
|
2 <3 |
25
|
0.57
|
0.57
|
|
Total ORFs with phenotypes 673 Total Homozygotes screened 4401 % of ORFs with phenotypes 15.29 |
||||
|
*1 stands for wild-type (WT) phenotype; |
||||
References:
Wach, A., Brachat, A., Pohlmann, R. & Philippsen, P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793-1808 (1994).
Shoemaker, D., Lashkari, D.A., Morris, D. , Mittmann, M. & Davis, R.W. Quantitative phenotypic analysis of yeast deletion mutants using a highly parallel molecular bar-coding strategy. Nature Genet 14, 450-456 (1996).
Gietz, D., St. Jean, A., Woods, R. A. & Schiestl, R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20, 1425 (1992).
Gietz, R.D., Schiestl, R.H., Willems, A.R. & Woods, R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355-60 (1995).
Hughes, T., et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nature Genetics 25, 333 - 337 (2000).
Brachmann, C.B., et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115-132 (1998)