
![]()
![]()
![]()
![]()
![]()
Protocols &Technical Information
![]()
![]()
![]()
Yeast Deletion Database (consortium members only)
![]()
Introduction
Materials & Methods
Replacement Cassette Module
Deletion Mutant Construction
Strain Confimation
Altered Cell Morphologies
A PCR-generated (Baudin 1993; Wach 1994) deletion strategy was used to systematically replace each yeast open reading frame from its start- to stop- codon with a KanMX module and two unique 20mer molecular bar codes. The presence of the tags can be detected via hybridization to a high-density oligonucleotide array, enabling growth phenotypes of individual strains to be analyzed in parallel.
Nearly all ORFs larger than 100 codons were disrupted; highly similar ORFs were not attempted (~3%). Four different Yeast Knock-Out (YKO.'s) collections have been generated: haploids of both mating types, homozygous diploids for non-essential genes, and heterozygous diploids, which contain the essential and non essential ORFs.
Approximately 5% of the yeast genome was not knocked out for various reasons. ORFs that were not unique due to gene duplication or contained regions of high sequence similarity were not attempted. Additionally, a number of ORFs were unsuccessfully deleted for unknown reasons; ~62% of these ORFs have no known biological function. For a list of these ORFs, click here.
The YKO collection of gene disruption mutants is an unparalleded resource for the scientific community
Each deletion "cassette" was constructed using two sequential
PCR reactions.
In the first amplification, 74bp UPTAG
and 74bp DNTAG primers amplify
the KanMX gene from pFA6-kanMX4
DNA whose KanMX expression confers dominant
selection of geneticin to yeast. These primers consist of (5' to 3'):
18 bp of genomic sequence that flank either the 5' or 3' end of the ORF
(directly proximal and distal to the start and stop codons respectively),
18 and 17 bp of sequence common to all gene disruptions (U1:
5'-GATGTCCACGAGGTCTCT-3' or D1:
5'-CGGTGTCGGTCTCGTAG-3'), a 20 bp unique sequence (the 'molecular bar-code'
TAG) and 18 and 19 bp of sequence, respectively, homologous to the KanMX4
cassette (U2: 5'- CGTACGCTGCAGGTCGAC-3'
or D2: 5'-ATCGATGAATTCGAGCTCG
-3')
In the second PCR reaction, two ORF specific 45-mer oligonucleotides (UP_45 and DOWN_45) are used to extend the ORF specific homology to 45 bp, increasing the targeting specificity during mitotic recombination of the gene disruption cassette.
The U1/D1 and U2/D2 sequences being common to all the deletion strains are used to amplify the 5' and 3' "molecular bar-codes" respectively via PCR for subsequent identification and analysis. The presence of the two tags (UPTAG and DNTAG) increases the quality of the hybridization data from the oligonucleotide arrays by adding redundancy. Early in the project one tag (UPTAG) was used exclusively; approximately 3.2 % of the strains harbor only one unique UPTAG sequence.
For each ORF, a minimum of two independent deletion mutants needed to
be produced. A standard Lithium-Acetate transformation
protocol (Gietz 1992; Gietz 1995) was used to introduce the gene disruption
cassette into diploid yeast cells (BY4743: MATa/a
his3D1/his3D1 leu2D0
/leu2D0 lys2D0/LYS2
MET15/met15D0 ura3D0
/ura3D0) followed by selection of colonies
on G418 containing agar plates. The resulting tranformants were sporulated
(Brachmann 1998) and haploids of both mating types, MATa and MATa,
were recovered from the tetrads. Dissection of tetrads from a heterozygous
diploid that gave segregants as 2 viable : 2 dead were deemed "essential"
and a second independent disruption was selected (B isolate). Mating of
the confirmed haploid deletion strains result in the homozygous diploid.
Approximately 30% of the gene disruptions were constructed by direct transformation
of MATa (BY4741: MATa his3D1 leu2D0
met15D0 ura3D0 )
and/or MATa (BY4742 MATa
his3D1 leu2D0 lys2D0
ura3D0) haploids. Mating of two independently
constructed haploid mutants generated homozygous diploids.
A second attempt was made to delete ORFs that were not successfully deleted in the first attempt. The success rates for Round 1 and 2 deletion attempts were 92% and 74% respectively. Genes not successfully deleted after the second round were attempted again using an additional pair of longer (63 mer) primers (UP_90 and DOWN _90) that extended the sequence flanking the ORF to be deleted to 90 bp. About 10% of the collection was attempted using such primers; the success rate for these disruptions (rounds 3 and 4) was >97%.
During construction of the YKO collection, two
problems had to be avoided.
First, a significant number of the primary heterozygous diploid transformants
carried recessive mutations unlinked to the gene deletion. These were
apparent in tetrads with lethal or slow growth mutations that segregated
independently of the KanMX gene. These
mutations are likely induced during the DNA transformation procedure,
which is known to be mutagenic. Mutants that behaved this way (about 6.5%
of the heterozygous KanMX-containing primary transformants, estimated
from a sample of 819 mutants), were discarded.
Second, a substantial fraction of the haploid deletion mutants were found to carry a wild-type copy of the gene to be deleted, in addition to the correct deletion mutation as confirmed by the appropriate PCR tests. Such cases are likely due to aneuploidy: duplication of all or part of the chromosome. They comprised about 1% of the heterozygous KanMX-containing primary transformants (estimated from a sample of about 1300 mutants), and upon identification through PCR they were discarded. (Hughes, 2000; B. Dujon, personal communication; our observations,described).
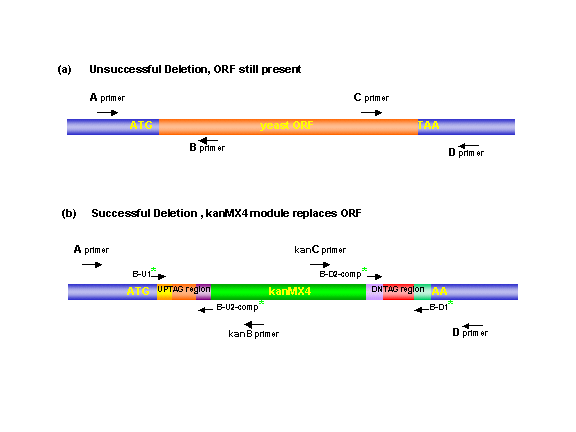
The correct replacement of the gene with KanMX
was verified in the mutants by the appearance of PCR products of the expected
size using primers that span the left and right junctions of the deletion
module within the genome. Four ORF-specific confirmation
primers (A, B, C, and D primers) were chosen for each ORF disruption.
The "A" and "D" primers were positioned 200-400 bp from the start and
stop codons of the gene, respectively. The "B" and "C" primers were located
within the coding region of the ORF and, when used with the A or D primers,
gave product sizes between 250-1000 bp. The "KanB"
(5'-CTGCAGCGAGGAGCCGTAAT-3') and "KanC"
(5'-TGATTTTGATGACGAGCGTAAT-3') primers are internal to the KanMX4 module.
For haploid or homozygous isolates, the junctions of the disruption were verified by amplification of genomic DNA using primers "A" and "KanB" and primers "KanC" and "D". Deletion of the ORF was verified by the absence of a PCR product using primers "A" with "B" and "C" with "D". In the case of heterozygous strains a successful deletion was indicated by the additional appearance of a wildtype-sized PCR product in reactions 3, 4 and 5. Finally, each deletion mutant was checked for a PCR product of the proper size using the primers flanking the gene.
In addition, each strain background was checked for the appropriate
auxotrophic markers and mating capabilities.
For more detailed protocols see: Deletion Project Primers, Strain Verification Criteria, PCR and Sporulation Protocols.
Screening the deletion mutants for altered cell morphologies:
Deletion strains were grown individually at 30°C with 0.8ml liquid YPAD in 96-well microtiter plates with shaking (Dot Scientific, U.S.A.); each well contained one 3.5 mm glass bead to facilitate mixing. The cells were grown to stationary phase, then diluted and grown to mid-log-phase (at least six generations). Cells were fixed by the addition of formaldehyde to a final concentration of 3.7%, incubated for one hour at 30°C, washed with PBS, resuspended in PBS, and examined by phase-contrast and differential interference contrast microscopy.
The mutants were scored using a scale of 1-4 (see table and legend
below) and grouped into seven classes: elongated, round, small, large,
football-shaped, clumpy and other. "Other," being defined
as a deletion mutant having 3 or more distinct phenotypes. Cells that
exhibited a "chain" or "branched" phenotype were
also catergorized as "other."
To see a list of all the strains screened, see the Cell
Morphology Screen Table.(Strains that are not yet screened are
designated as "ND.")
To see the morphologies categorized by their functional classes, click
here.
Morphological Screen Results
|
Phenotype
|
Score*
|
No.
|
%
|
%
|
|
Elongate
|
2 <3 |
62 57 |
1.41 1.30 |
2.70
|
|
Round
|
2 <3 |
104 98 |
2.36 2.23 |
4.59
|
|
Small
|
2 <3 |
87 75 |
1.98 1.70 |
3.68
|
|
Large
|
2 <3 |
69 76 |
1.57 1.73 |
3.29
|
|
Football
|
2 <3 |
53 59 |
1.20 1.34 |
2.54
|
|
Clumpy |
2 <3 |
10 14 |
0.23 0.32 |
0.55
|
|
Other
|
2 <3 |
25
|
0.57
|
0.57
|
|
Total ORFs with phenotypes 673 Total Homozygotes screened 4401 % of ORFs with phenotypes 15.29 |
||||
|
*1 stands for wild-type (WT) phenotype; |
||||
